The FDA Just Approved the First Generic Nasal Spray to Reverse Opioid Overdoses
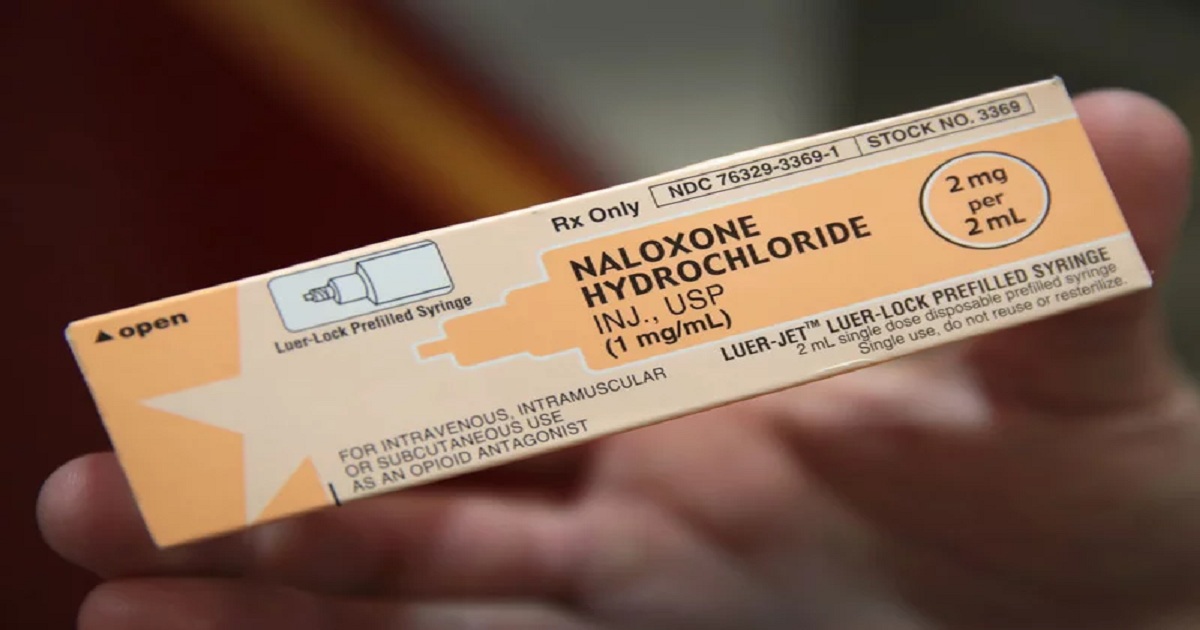
The Food and Drug Administration (FDA) on Friday announced that it granted final approval to the first generic naloxone hydrochloride nasal spray, which can be used to reverse opioid overdoses. The approval is part of the FDA’s wider effort to make tools for stopping or preventing opioid overdoses more accessible and widely used. The agency is also working with drug companies to bring over-the-counter versions of naloxone to market, and is prioritizing the approval of other generic naloxone products. Friday’s announcement, which makes official a tentative approval previously granted to Teva Pharmaceuticals, means there will soon be a generic alternative to Narcan, the widely used naloxone nasal spray sold by Emergent BioSolutions.