Taking a Closer Look at Drug Delivery
Technology Networks | October 31, 2019
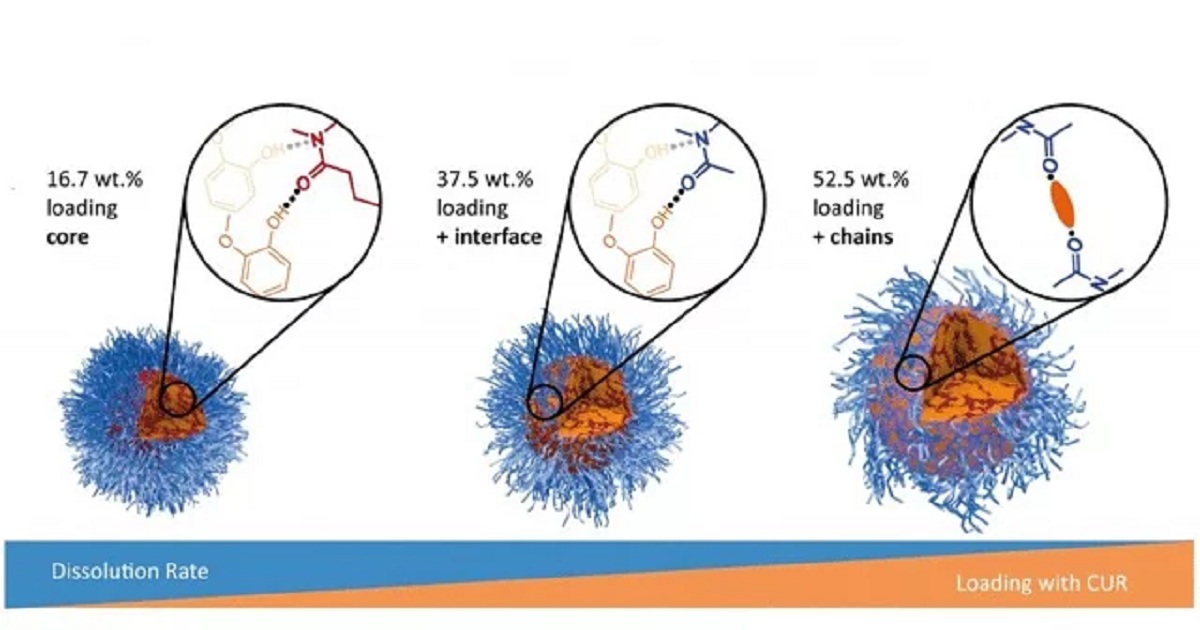
Nanocapsules and other containers can transport drugs through a patient's body directly to the origin of the disease and release them there in a controlled manner. Such sophisticated systems are occasionally used in cancer therapy. Because they work very specifically, they have fewer side effects than drugs that are distributed throughout the entire organism. This method is known in science as drug delivery. Chemistry professor Ann-Christin Pöppler from Julius-Maximilians-Universität (JMU) Würzburg in Bavaria, Germany, is convinced that this method still has great development potential. She analyzes the molecular capsules that enclose drugs like a container and transport them to the site of action: "My group wants to understand in as much detail as possible how the container molecules and the active substances arrange and what properties result from this," she says. The junior professor is mainly investigating polymeric micelles. These consist of many chains of molecules, which assemble into spherical structures. Such micelles are already on the market as drug containers. They are used in cancer therapies as well as in cosmetic products such as make-up remover lotions. When they come into contact with fat-soluble substances, they arrange themselves on their surface and at the end surround them like a coat of hair. This forms a container with a "water-loving" outer shell and a "fat-loving" core. "Little is known about the molecular origin of the properties of these structures," says Pöppler. In the scientific journal Angewandte Chemie, the researcher and co-authors from JMU recently described an effect that is important for the design of future drug delivery systems: If increasing amounts of active ingredients are packed into the polymeric micelles, their dissolution suffers - the release of the active ingredients then becomes increasingly difficult.