Mylan, Biocon copy inches closer to challenging Sanofi's long-suffering Lantus
Questex LLC | December 13, 2018
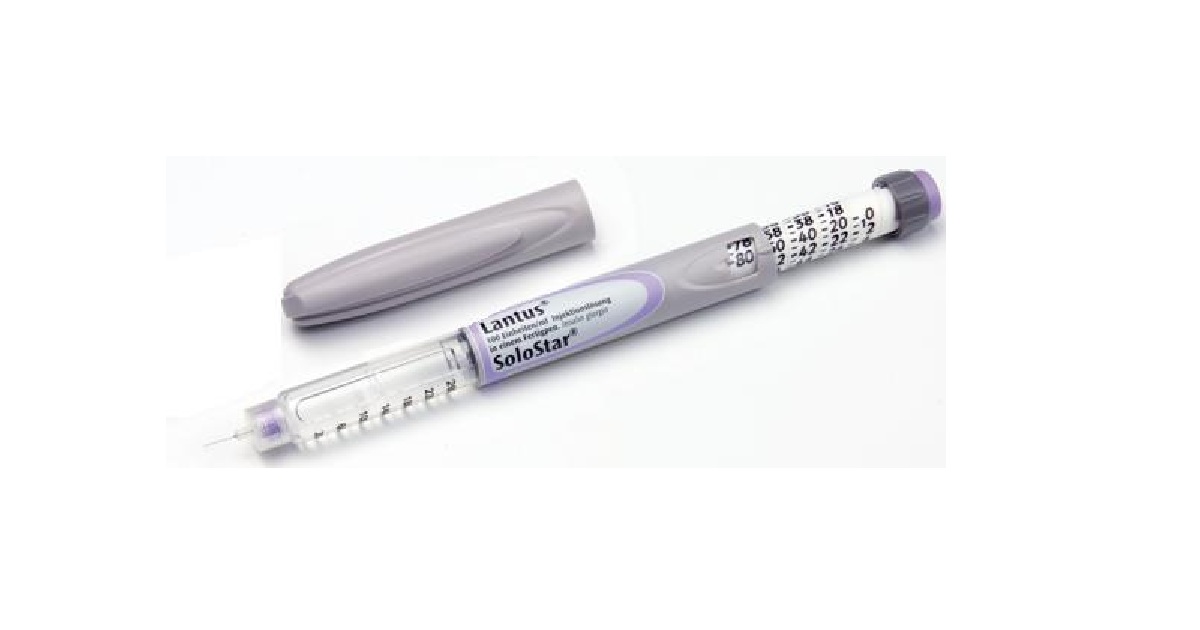
Sanofi’s Lantus is already facing off against generic competition from Eli Lilly and Boehringer’s Basaglar, but now Mylan is adding to the pressure by prevailing in a patent challenge at the U.S. Patent and Trademark Office. The PTO’s Patent Trial and Appeal Board invalidated two Lantus formulation patents, knocking down one barrier to market for Mylan and its partner Biocon as their generic awaits a decision at the FDA. The partners still face a patent fight with Sanofi in court; Lilly and Boehringer launched their copycat, Basaglar, in 2016 under a patent settlement with the French drugmaker.
Mylan's copy will be gunning for Sanofi’s billions in Lantus sales, but the once-dominant basal insulin has already been hit hard by Basaglar and other competition. Lantus revenue plummeted 31.7% in the third quarter to €419 million, thanks to lower net prices and coverage changes in Medicare Part D. In fact, the pricing pressure on Lantus and its fellow basal insulins—including still-on-patent brands—actually convinced Merck to discontinue development on its own copycat back in October. At the time, a Merck spokesperson said the company had assessed the market outlook, including “anticipated pricing and cost of production,” before nixing the deal.