More Patients Can Now Be Treated With New SAPIEN 3 Ultra Transcatheter Aortic Valve
Medgadget | January 02, 2019
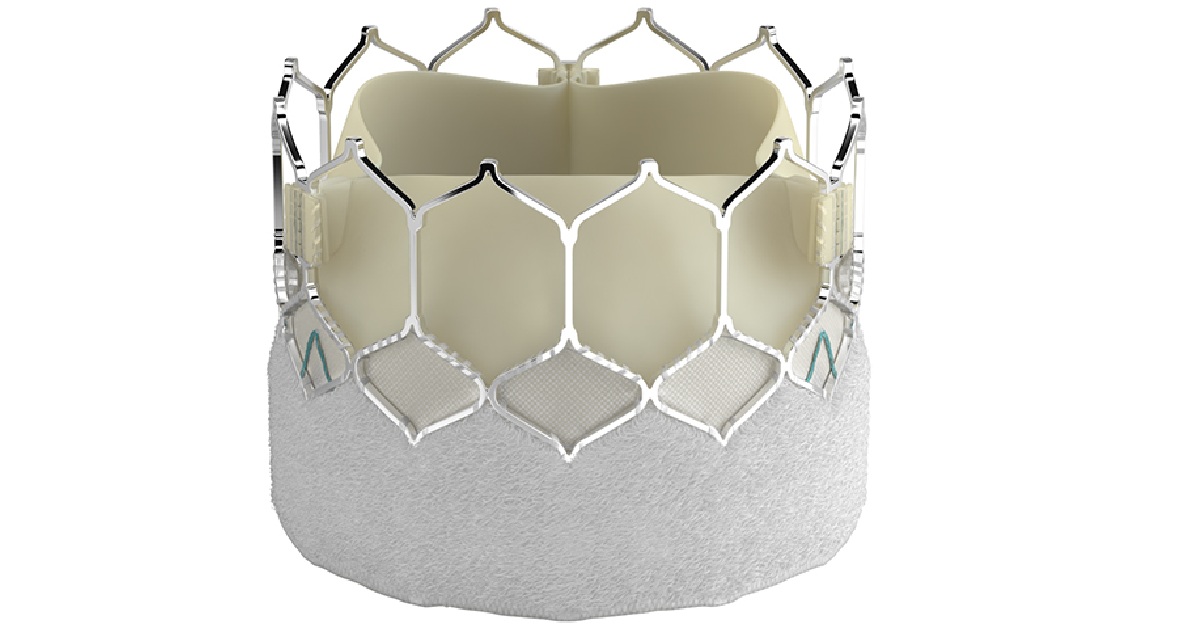
Edwards Lifesciences received FDA approval to introduce its SAPIEN 3 Ultra transcatheter aortic heart valve in the U.S. Specifically, the device is indicated for cases of severe, symptomatic aortic stenosis identified to be at the intermediate or greater risk of open-heart surgery. The SAPIEN 3 Ultra has some modifications that make it appropriate to a wider range of patients that would otherwise not be able to get a transcatheter aortic valve. And a brand new delivery system and sheath make the implantation procedure a bit easier on the physicians. The valve features an extended skirt on its exterior that helps to avoid leakages and a 14-French low profile sheath. The delivery system uses an “on balloon” design that doesn’t require the surgeon to have to manually align the artificial valve with the native anatomy. This helps to speed up procedures, reducing risks for patients and saving money for hospitals.