ViiV to develop bNAb candidate to treat and prevent HIV
Pharmaceutical Technology | November 22, 2019
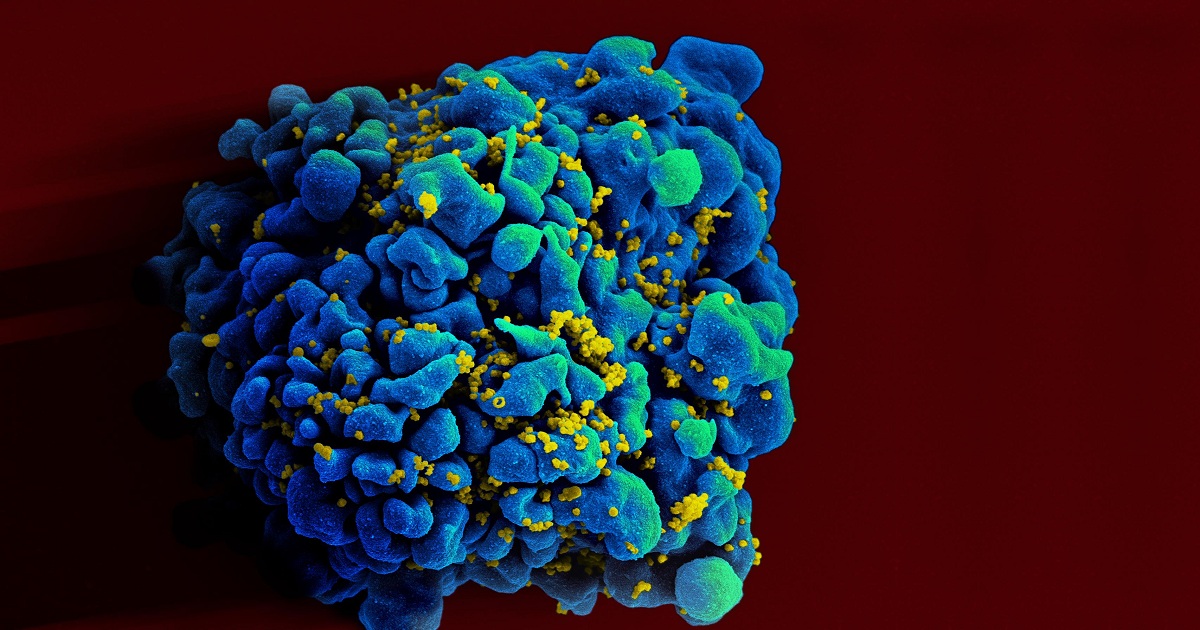
ViiV Healthcare has agreed to develop an investigational broadly neutralising antibody (bNAb) N6LS to treat and prevent human immunodeficiency virus 1 (HIV-1) infection. Founded in 2009 by GlaxoSmithKline (GSK) and Pfizer, ViiV focuses on developing therapies against HIV. Japanese pharmaceutical firm Shionogi also holds a stake in ViiV. The development of the bNAb candidate falls under an exclusive licensing agreement between GSK and the National Institutes of Health (NIH)’s National Institute of Allergy and Infectious Diseases (NIAID). N6LS binds to the gp120 site on the surface of the virus and prevents its entry into healthy CD4+ T-cells, a type of immune system cells. The process is meant to stop HIV replication and potentially prevent HIV transmission. NIAID’s Laboratory of Immunoregulation and Vaccine Research Center (VRC) researchers discovered and conducted initial development of N6LS.