Pfizer is target of federal civil probe into EpiPen malfunctions
Questex LLC | March 01, 2019
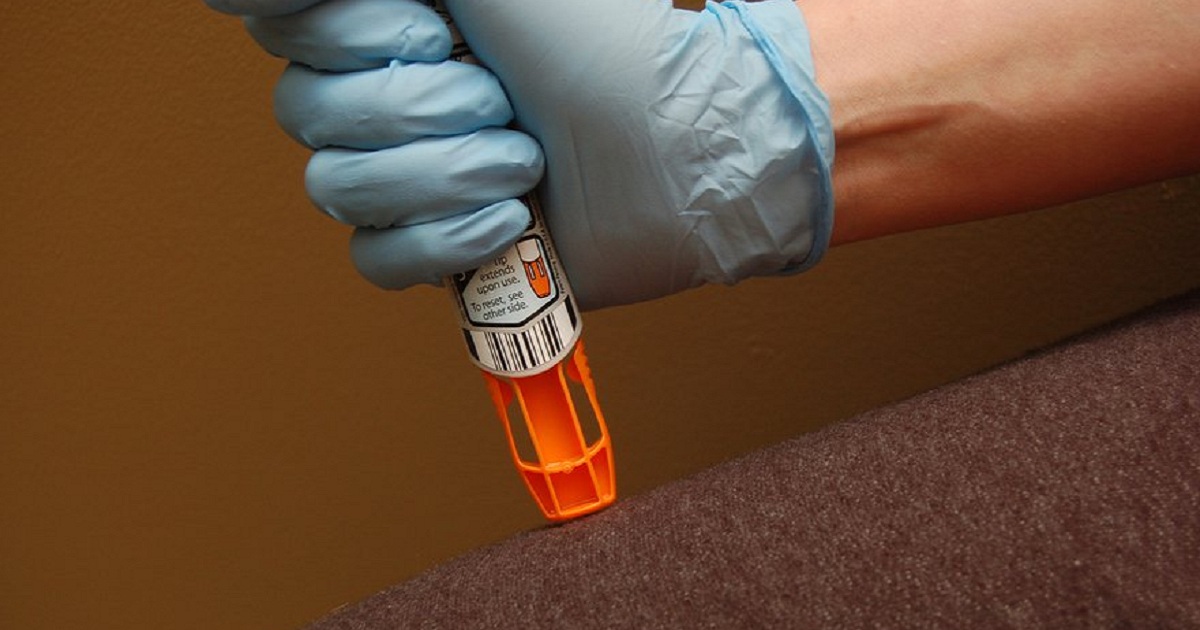
Problems for the troubled Pfizer unit that makes EpiPen injectors for Mylan have opened on a new front. The U.S. Attorney for the Southern District of New York is now digging into the issues of why Pfizer continued to produce pens after receiving dozens of complaints that many were failing during life-and-death emergencies. Pfizer disclosed in a public filing today that U.S. attorney in February made a civil investigative demand for records and information related to “alleged quality issues involving the manufacture of auto-injectors at our Meridian site.” Pfizer said it will be producing records in response.
The company on Friday declined to say anything beyond the statement in the filing or to say anything about the status of remediation of the plant in St. Louis, Missouri, that makes the injectors used to treat anaphylactic shock from allergic reactions.
Pfizer was quick to defend its operations in 2017 when it received the FDA warning letter that shredded the operation for failing to thoroughly investigate dozens of complaints that EpiPen and EpiPen Jr. products had failed, some of them "during life-threatening emergencies, including some situations in which patients subsequently died.”