Cell therapy produces encouraging first results in eye trial
Pharmaphorum Media Limited | February 22, 2019
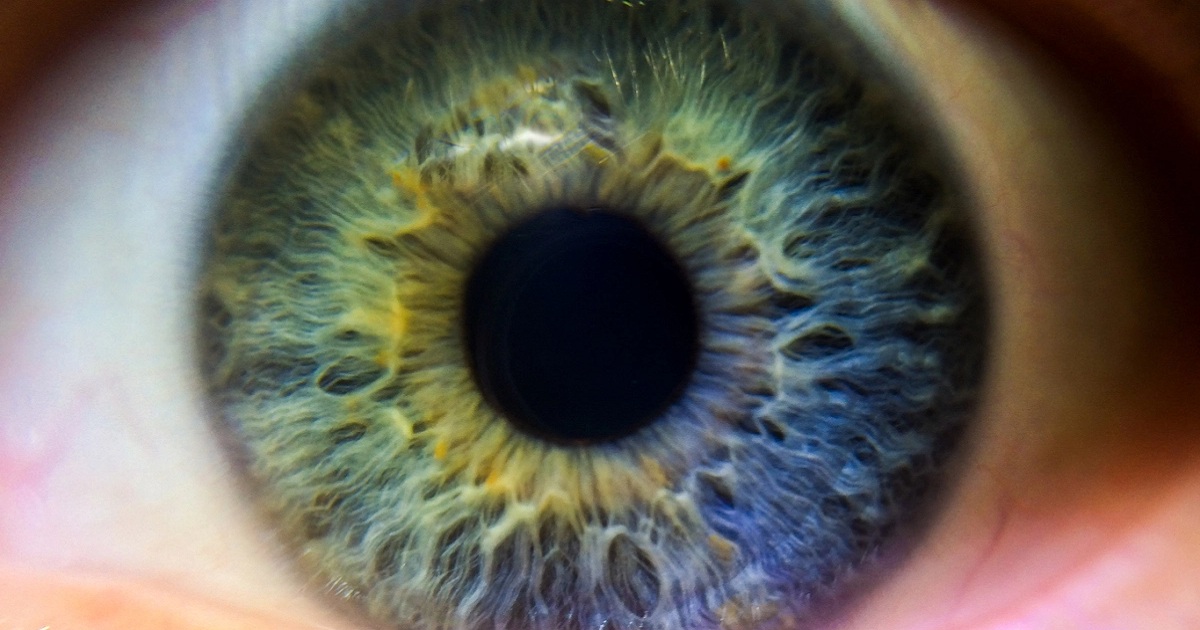
UK-based biotech ReNeuron has announced encouraging results from an early-stage trial of its cell therapy for the rare blindness-causing disease, retinitis pigmentosa (RP). The only results so far are from three subjects in the first cohort in phase 2 of a phase 1/2 trial of the disease. But this therapy is based on a dose of human retinal progenitor cells, and if approved could be a competitor to Spark/Novartis’s Luxturna (voretigene neparvovec). Unlike the already-approved gene therapy Luxturna, ReNeuron’s cell-based therapy would not be limited to patients with a disease caused by a certain mutation.
Luxturna is based around a virus delivering genetic material to correct a genetic sight defect, while ReNeuron’s therapy involves human retinal progenitor cells (hRPC) being embedded in the back of the eye. This creates new photoreceptors – and represents the first time new photoreceptors have ever been created in humans. ReNeuron said all three subjects in the first cohort of the phase 2 part of the trial have demonstrated a significant improvement in vision at follow-up compared with their pre-treatment baseline and compared with the untreated eye.