Pfizer’ Talzenna leads latest CHMP highlights
pharmatimes | April 29, 2019
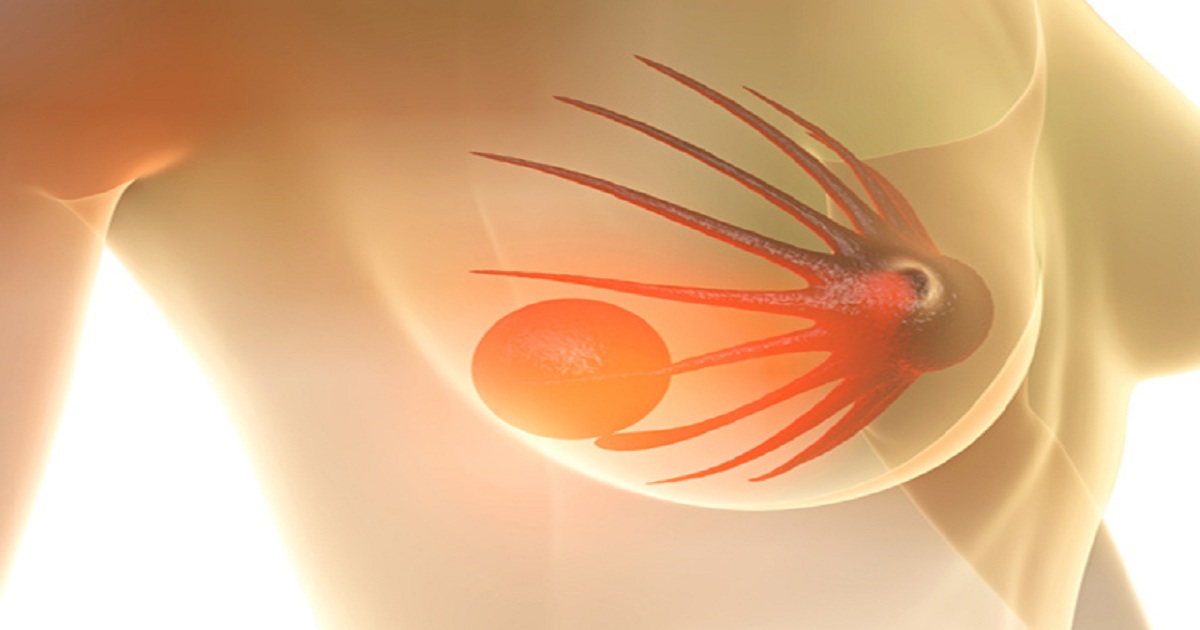
Pfizer’s PARP inhibitor, Talzenna (talazoparib), has been given a positive opinion by the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) for adults with germline breast cancer susceptibility gene (gBRCA)1/2-mutations, heading up April’s recommendations list. Thirteen medicines were recommended for approval at the Committee’s April 2019 meeting, including two orphan medicines; Novo Nordisk’s Esperoct (turoctocog alfa pegol), for the treatment and prophylaxis of bleeding in patients 12 years and above with haemophilia A; and Alexion’s Ultomiris (ravulizumab), for the treatment of adult patients with paroxysmal nocturnal haemoglobinuria.