HIV Drug Delivery Made Easier with Long-Acting Implant
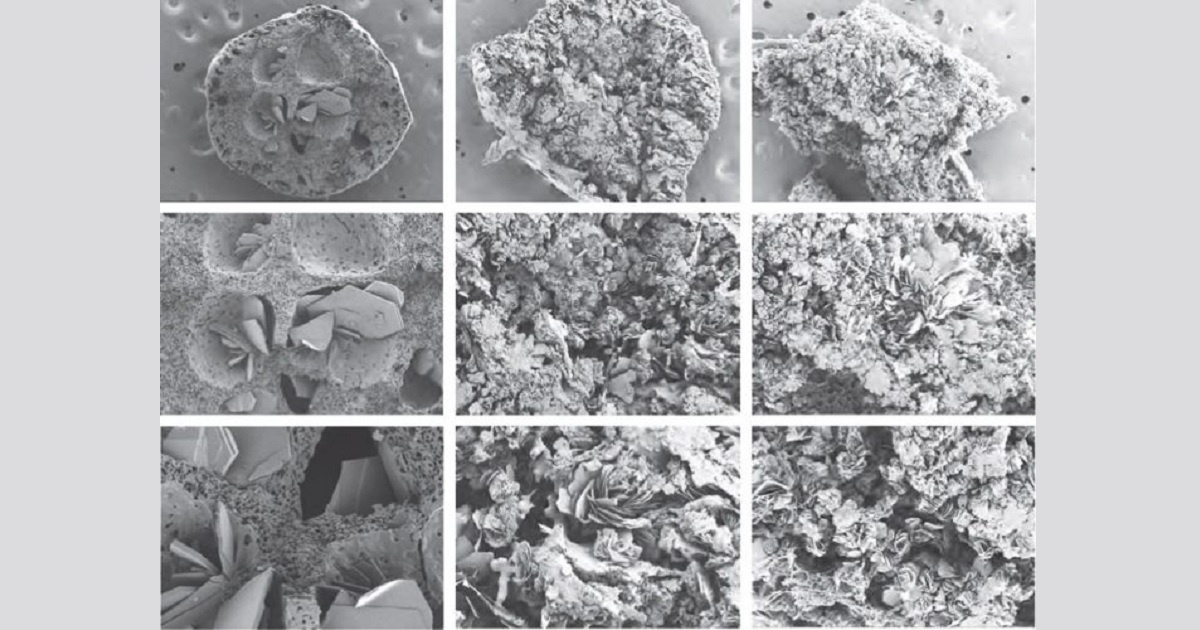
Adherence to a strict HIV regimen is an essential part of effective HIV treatment. Medication should be taken every day, at specific times of the day, and with or without certain kinds of food. Making this process easier would, undoubtedly, increase medication adherence and improve the efficacy of current HIV treatment plans. Now, a group of researchers from the University of North Carolina (UNC) at Chapel Hill reports an ultra-long-acting tunable, biodegradable, and removable polymer-based delivery system that offers sustained drug delivery for up to one year for HIV treatment or prophylaxis. The authors write that the goal of this seven-year study in animals was to develop a delivery system that can offer ultra-long-acting drug release by (1) providing flexibility in the choice of active ingredient, (2) sustained release for weeks or months, (3) the ability to be surgically removed in case of an allergic or adverse reaction, (4) and the ability to integrate multiple drugs.