Grifols donations of blood clotting factor helping people with hemophilia globally
pharmiweb | April 15, 2019
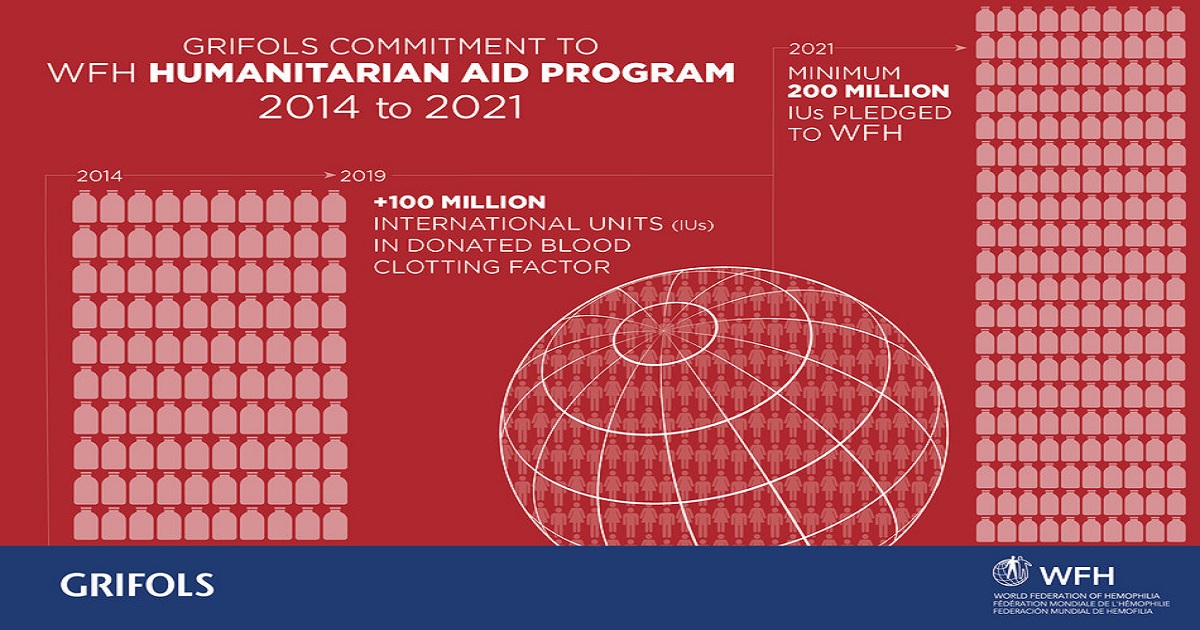
Grifols, one of the world's three top providers of plasma-derived medicines to treat life-threatening diseases, today announced that its long-term global initiative to providing treatment to people with hemophilia in developing regions has surpassed 100 million international units (IUs) in donated blood clotting factor, a protein in blood that controls bleeding. Grifols is working to enhance the lives of the roughly 100,000 people around the world who have hemophilia but receive little or no treatment. The company is halfway into an eight-year initiative in which it has pledged a minimum of 200 million IUs to the World Federation of Hemophilia (WFH) Humanitarian Aid Program through 2021. According to the WFH, Grifols full donations will secure a projected average of 10,300 doses to treat acute bleeds in 6,000 patients per year in developing regions through 2021. Over the last two years, these donations have treated more than 5,000 patients and more than 10,000 acute bleeds. "World Hemophilia Day reminds us that there are tens of thousands of people who live with hemophilia in many countries and don't have access to care and treatment," said Victor Grifols Deu, co-CEO of Grifols. "Here at Grifols we're proud to partner with the WFH in its dedication to helping ensure that hemophilia patients in developing regions receive critical and often life-saving medicines. It's about people helping people." The company has also created the Grifols Humanitarian Awards in Hemophilia to encourage health care providers, medical and paramedical centers as well as hemophilia societies to support education and access to treatment for the approximately 400,000 people in developed and developing regions who have this inherited condition. Celebrating the first edition of the award, Grifols will award four €50,000 grants to eligible proposals, naming the recipients in early 2020. The Grifols Humanitarian Awards complement the company's Martín Villar Hemostasis Awards that support the investigation of hemophilia and von Willebrand disease. Since establishing this award in 2007, Grifols has conferred approximately €640,000 euros to 28 researchers.