DRC to administer second vaccination to tackle Ebola outbreak
Pharmaceutical Technology | September 23, 2019
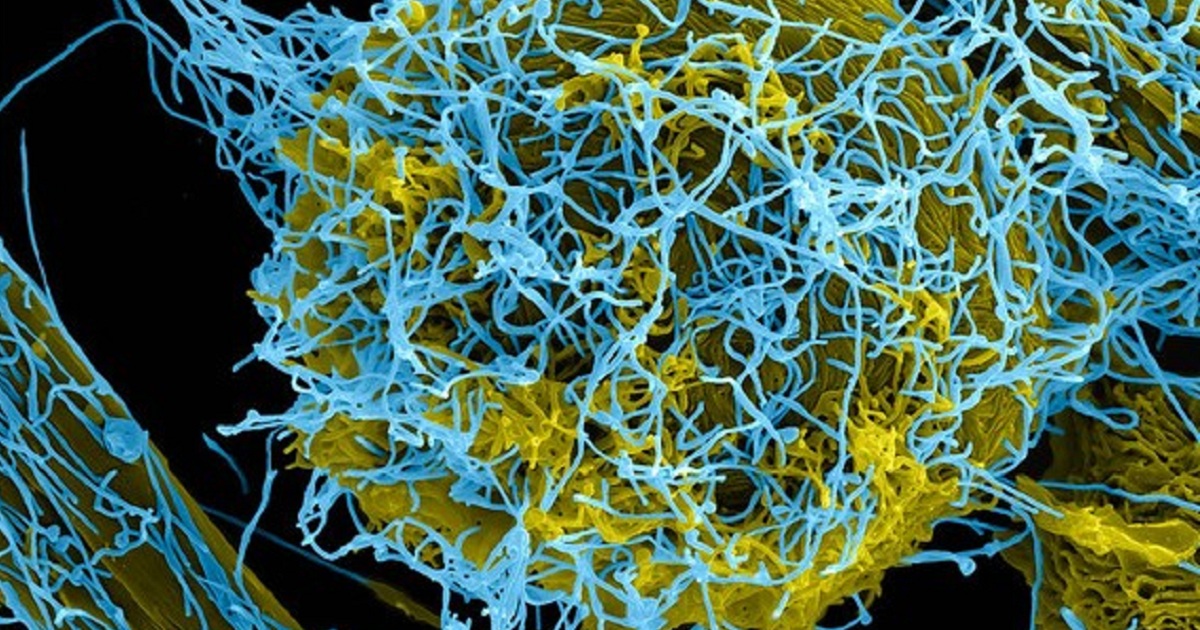
The World Health Organization (WHO) has announced that the health authorities of the Democratic Republic of Congo (DRC), which is currently tackling a serious Ebola epidemic, will be providing vaccination to at-risk populations with a second product in addition to the current use of Merck’s rVSV-ZEBOV-GP. The vaccine chosen for the second vaccination is manufactured by Johnson & Johnson (J&J) and will be provided in two doses 56 days apart. It will administered to at-risk people in areas yet to see active Ebola transmission. WHO director-general Dr Tedros Adhanom Ghebreyesus said: “The DRC authorities, in deciding to deploy the second experimental vaccine to extend protection against this deadly virus, have once again shown leadership and their determination to end this outbreak as soon as possible.” The organisation’s regional director for Africa Dr Matshidiso Moeti commented: “The evaluation of the second Ebola vaccine will help ensure that we have potentially an additional tool to prevent the expansion of the outbreak and also a potential tool to protect populations before outbreaks hit areas at risk.”