Big Pharma game heading for console release next month
pharmaphorum | November 19, 2019
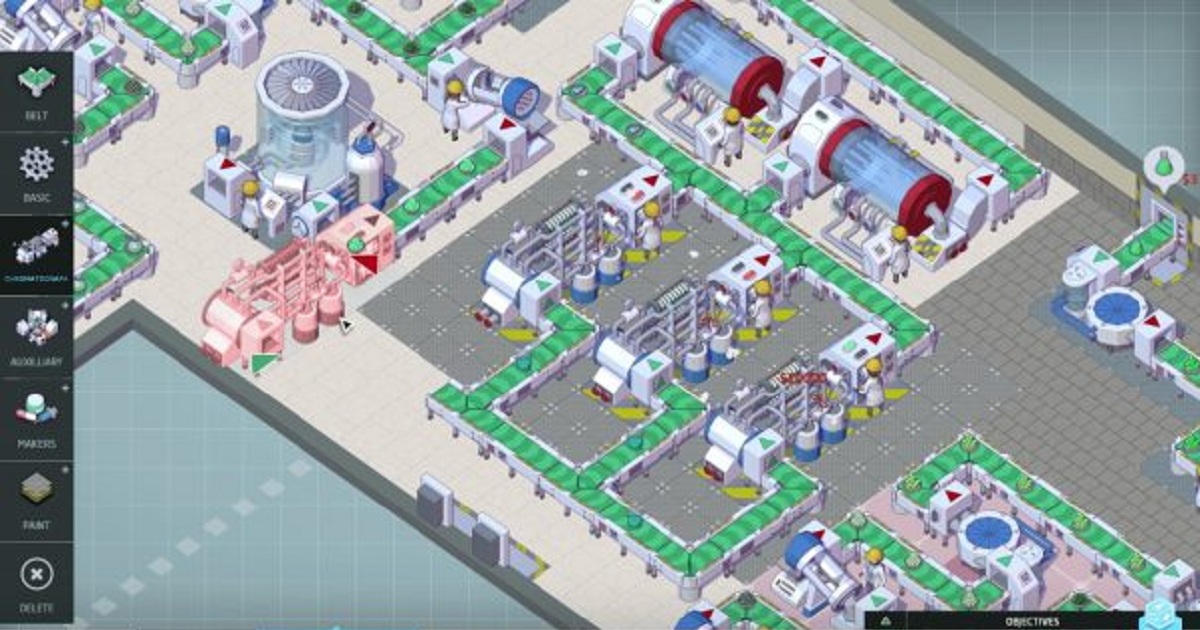
First launched as a PC game in 2015, Big Pharma will be available for all three major games consoles – Sony’s PlayStation 4, Nintendo Switch, and Microsoft Xbox One – from next month, according to publisher Klabater. The game – developed by Twice Circled and Positech Games – is described as “part business sim, part logistics puzzle,” and puts players in the hot seat of a biopharma start-up, trying to translate R&D discoveries into blockbuster brands and transform the company into a multinational corporation. Players start with an empty warehouse, and have to buy the equipment and technology to try to turn it into a profitable factory with multiple production lines, whilst also pumping cash into R&D and sending drug-hunting explorers around the world. The game includes a number of challenges and quests to test budding CEOs as they try to build their empire, and discover whether their business approach is closer to disgraced ‘pharma bro’ Martin Shkreli – currently incarcerated – or legendary biotech pioneer Henri Termeer.