AstraZeneca improves diabetes pen to maintain its market share
Pharmaphorum Media Limited | August 31, 2018
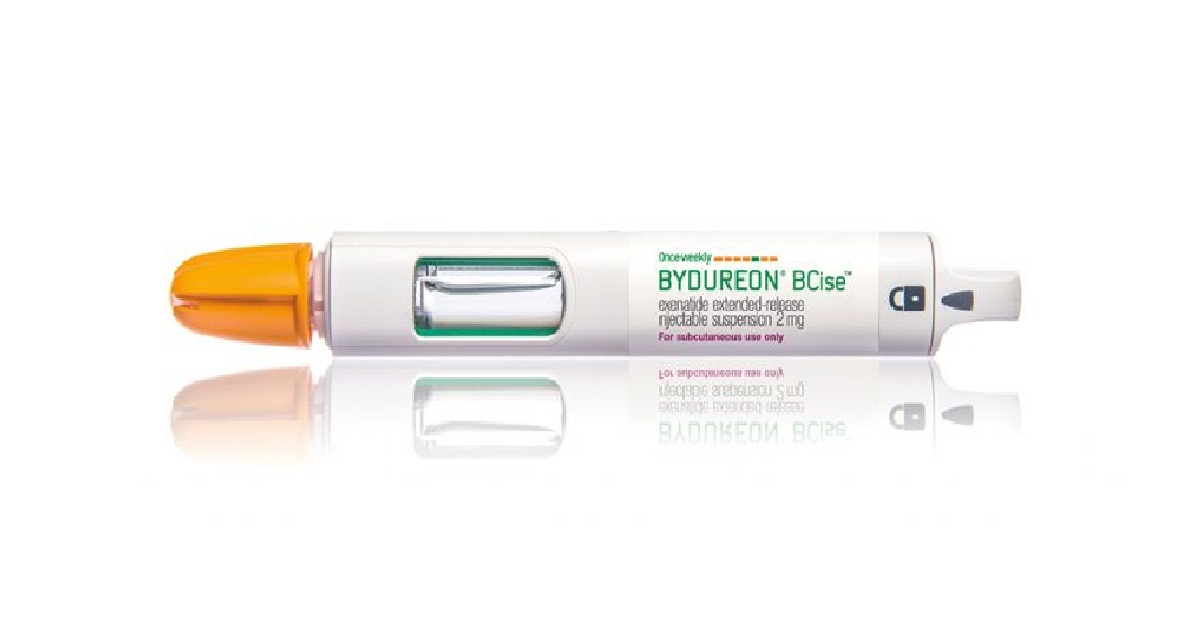
The European Commission (EC) has approved AstraZeneca’s Bydureon BCise, a new weekly formulation of established GLP-1 diabetes drug with the added benefit of glucose reduction, better weight loss results and a new injection pen. AstraZeneca announced that the EC green-lighted the new formulation of the once-weekly type-2 diabetes injection, which will be marketed as Bydureon BCise (exenatide 2mg prolonged-release suspension). The new authorisation has been granted for a single-dose, pre-filled pen device that requires no titration and is approved for use in combination with other glucose-lowering medicines, including basal insulin, to help improve glycaemic control together with diet and exercise.